Direct Reduction of Titanium Slag (DRTS)
Novel Ti-powder Production Route
A Novel Chemical Pathway for Production of Titanium Metal Powder
In the spring of 2014, Dr. Zak Fang and his team were awarded funding for their proposed Direct Reduction of Titanium Slag (DRTS) project through AARP-E of the Federal Government. This project focusses on understanding in detail the mechanism of the reactions involved, scaling up to a kilogram scale, and “de-risking” the project (if possible) from both a technical and a financial investment perspective through rigorous techno-economic analysis. The project is scheduled to run for three years.
Titanium metal is a highly coveted material for its high strength-to-weight ratio, relatively high melting point (compared with aluminum for example), resistance to corrosion, compatibility to the human body, and pleasing appearance. Titanium is also quite common in the earth’s crust, being the ninth most abundant element and the fourth most abundant metal, yet it remains highly underutilized. Although the Kroll and Hunter processes are effective in producing high purity titanium metal, their throughput is limited by the lengthy time required for each batch, and the high energy input needed. This batch processing restraint and the high energy inpute required, result in a high selling price for titanium sponge of over $10,000 per ton. It is expected that a reduction in titanium metal’s cost would result in an expansion of it’s uses in both current applications and new sectors that are now barred due to high cost.
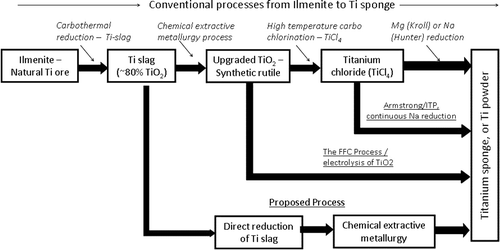
Preliminary laboratory-scale experiments showed the technical viability of the DRTS process on a gram scale. Based on these initial laboratory-test results, a savings of approximately 62% was calculated to exist for the production costs of titanium metal using the DRTS process over other conventional processes such as the Kroll or Hunter process. The DRTS process is able to achieve these savings because it by-passes very energy intensive processes currently in use, as outlined in Fig 1 below.
 The most unique part of DRTS is the direct reduction step using magnesium hydride
to form titanium hydride from upgraded titanium slag (UGS). This reaction is thermodynamically
favorable based on the thermodynamic modeling results (HSC chemistry 5.11). The viability
of the DRTS process is due in part to the unusually high vapor pressure of magnesium
at 500oC which helps to drive the reduction process, and the insolubility of TiH2
in water (as well as its resistance to moderate acids and bases used in the leaching
steps). Eutectic salts are also used to facilitate faster kinetics. Removal of impurities
after reduction is accomplished through a series of leaching steps, after which dehydrogenation
is carried out to leave a final product of titanium metal powder. The process uses
industrially proven equipment and techniques.
The most unique part of DRTS is the direct reduction step using magnesium hydride
to form titanium hydride from upgraded titanium slag (UGS). This reaction is thermodynamically
favorable based on the thermodynamic modeling results (HSC chemistry 5.11). The viability
of the DRTS process is due in part to the unusually high vapor pressure of magnesium
at 500oC which helps to drive the reduction process, and the insolubility of TiH2
in water (as well as its resistance to moderate acids and bases used in the leaching
steps). Eutectic salts are also used to facilitate faster kinetics. Removal of impurities
after reduction is accomplished through a series of leaching steps, after which dehydrogenation
is carried out to leave a final product of titanium metal powder. The process uses
industrially proven equipment and techniques.
Fig 1. Conventional vs DRTS: Processing Routes for Production of Titanium Metal
An overview of the chemical pathway of DRTS is given below in Fig 2. This schematic shows the reduction and leaching steps and the role that each of these steps play in preparing Ti-metal powder from Ti-slag.
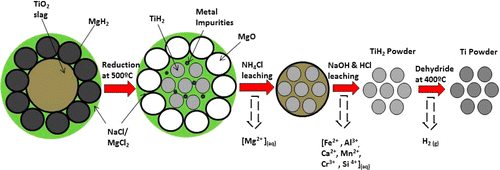
Fig 2. DRTS Process Schematic, as Published in Journal of American Chemical Society (JACS)
References:
- Z. Zak Fang, Scott Middlemas, Peng Fan, and Jun Guo, “A Novel Chemical Pathway for Producing Low Cost Ti by Direct Reduction of Ti-slag”, Journal of American Chemical Society , 2013, 135 (49), pp 18248–18251
- Z. Zak Fang, et al. “A Novel Chemical Pathway for Titanium Production to Drastically Reduce Cost.” Proposal to AARP-E. 2013
Hydrogen Sintering and Phase Transformation
Production of fully dense Ti-alloys with wrought-like microstructures, from Ti-powders
Over the past five years, our group has developed a new approach for manufacturing high performance components of Ti or Ti alloys via a novel hydrogen sintering process. The new process is called hydrogen sintering and phase transformation (HSPT). HSPT is capable of producing fully dense Ti alloys with wrought-like microstructures and mechanical properties without requiring energy-intensive thermomechanical processing (TMP) that is compulsory to other Ti alloy production techniques. We have also developed new techniques for near-net-shape (NNS) fabrication of Ti components based on the cold isostatic press (CIP) process as well as traditional die pressing. Currently, our group is collaborating with U.S. Army Research Laboratory (ARL) to produce Ti components for military applications. Additionally, our group is collaborating with AMETEK, a leader in PM Ti powder and parts production. Furthermore, the group has collaborated with the Orthopedic Research Laboratory at the University of Utah to develop Ti based advanced implants for a range of medical applications that demand high strength, light weight, and biocompatibility.
Ti alloys are attractive for many applications due to their unique combination of high strength, low density, corrosion resistance, and biocompatibility. Currently, wrought processing is the state of the art for producing high performance Ti alloys. However, wrought processing requires multiple steps of energy-intensive thermomechanical processing (TMP) to both form the shape and produce the desired microstructure and corresponding mechanical properties. Additionally, many Ti components have high buy-to-fly ratios, meaning the amount of material lost during forming and machining is substantial. Because of these facts, wrought Ti is prohibitively expensive for most commercial applications. Powder metallurgy (PM) has long been regarded as a viable and promising approach for reducing the cost of Ti fabrication because of the NNS capability of PM processes. However, traditional PM processing produces Ti alloys with inferior mechanical properties due to the poor densification and the coarse microstructure that results from the high sintering temperature. The mechanical properties of traditional PM Ti can be improved by utilizing methods such as pre-alloying the powder, pressure-assisted sintering, and post-sintering TMP to remove porosity and refine the microstructure. However, these processes are energy-intensive and eliminate the economic benefit of PM when incorporated into a manufacturing process.
The key innovation of the HSPT process is the utilization of a partial pressure of H2 in the atmosphere during sintering. By doing so, the hydrogen content of the alloy may be controlled throughout the thermal cycle. The thermodynamics and kinetics of the phase transformations in the Ti(alloy)-H alloy systems are a function of temperature as well as hydrogen content. Therefore, HSPT is able to control the phase transformations as a function of both of these parameters [1,2]. By utilizing said phase transformations, HSPT is capable of producing an ultrafine grain (UFG) microstructure in the as-sintered state. Additionally, the UFG microstructure has sufficient internal energy to provide a driving force for phase transformations during subsequent heat treatments. This is a significant advantage over traditional wrought or PM processing. These traditional processes require TMP to produce the driving force required for microstructural refinement via phase transformations and recrystallization. However, TMP is inherently energy-intensive and the primary source of cost in these processes. Therefore, HSPT maximizes the performance-to-cost ratio of Ti alloys by producing high performance material without requiring costly processing techniques [3]. When HSPT is coupled with simple heat treatments, it can produce a range of wrought-like microstructures. Therefore, Ti alloys may be engineered via HSPT to have application-tailored mechanical properties without requiring TMP.
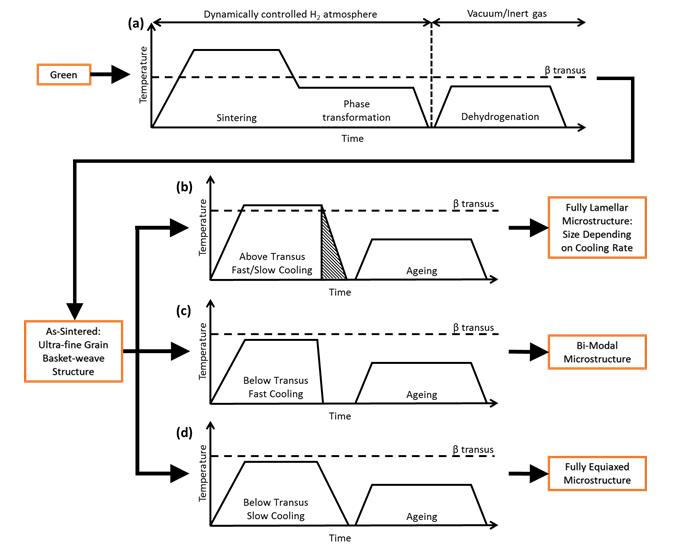
Fig 1. Schematic representation of a typical HSPT thermal profile for sintering and subsequent heat treatments.
A schematic representation of a typical HSPT sintering and dehydrogenation profile as well as typical heat treatments used in this research are shown in Figure 1. The HSPT sintering process (Figure 1-a) is a novel process developed by our team. However, the subsequent heat treatments (Figure 1-b through d) used to produce wrought-like microstructures are borrowed directly from wrought processing [4]. A detailed description of the three steps in a typical HSPT process shown in Figure 1-a is given below [1]:
Step I – Sintering: During the first step in the HSPT process, the material is brought to a temperature above the ß-transus (1000~1300 °C) and held for several hours. A dynamically controlled H2 partial pressure of 1~100 kPa is utilized to maintain hydrogen content within the alloy during sintering. Higher H2 partial pressures up to 1 MPa (10 bar) can be utilized to increase the hydrogen content during sintering, but this is not typically necessary. The H2 partial pressure is controlled by using either pure H2 at a controlled pressure or a mixture of H2 and inert gas. At these temperatures and hydrogen partial pressures, the material is entirely ß-Ti(H), ß phase with dissolved hydrogen. The high temperature and presence of dissolved hydrogen results in rapid densification kinetics, thereby facilitating densification over 99% relative density at comparatively moderate temperatures and hold times.
Step II – Phase Transformation: During the second step in the HSPT process, the microstructure of the alloy is refined via phase transformations in the Ti(alloy)-H alloy systems. The H2 partial pressure is dynamically controlled during this step as well. The thermodynamics (phase equilibria) and kinetics of the phase transformations are a function of both temperature and hydrogen content within the alloy. Therefore, the phase transformations that occur can be controlled as a function of both temperature and H2 partial pressure. Directly after sintering, the material is cooled within the furnace to a multi-phase field. This causes ß-Ti (BCC) to decompose into low temperature phases such as a-Ti (HCP), a2-Ti3Al (ordered HCP), and d-TiH2 (FCC). Nucleation of the low temperature phases refines the microstructure. Additionally, d-TiH2 (?TiH2 =3.75 g/cm3) is significantly less dense than metallic Ti (?Ti=4.51 g/cm3). Therefore, it is theorized that precipitation of the hydride phase may result in localized strain that can lead to recrystallization during subsequent thermal profiles.
Step III – Dehydrogenation: During this step, residual hydrogen is removed from the alloy by annealing at a temperature well below the ß-transus. Dehydrogenation may be performed under either vacuum or in an inert gas atmosphere with a sufficiently low H2 partial pressure. Experiments using either inert gas or low-vacuum (>10-1 Pa) typically result in hydrogen levels well below the ASTM standard of 150 ppm [5]. However, when high-vacuum is used (<10-2 Pa), hydrogen levels below 10 ppm are consistently achieved after dehydrogenation. Dehydrogenation may be performed directly following the phase transformation step in the same furnace, without first cooling the material. Conversely, this step may be performed in a separate dehydrogenation furnace. During dehydrogenation, further grain refinement is achieved due to phase transformations that result from hydrogen’s removal. Hydrogen is a strong ß stabilizer. Therefore, dehydrogenation causes d-TiH2 to decompose into ß, followed by ß decomposing into a and a2. Finally, it has been theorized that localized strain caused by the formation of d-TiH2 during the phase transformation step may result in recrystallization during dehydrogenation and subsequent heat treatments, as mentioned above.
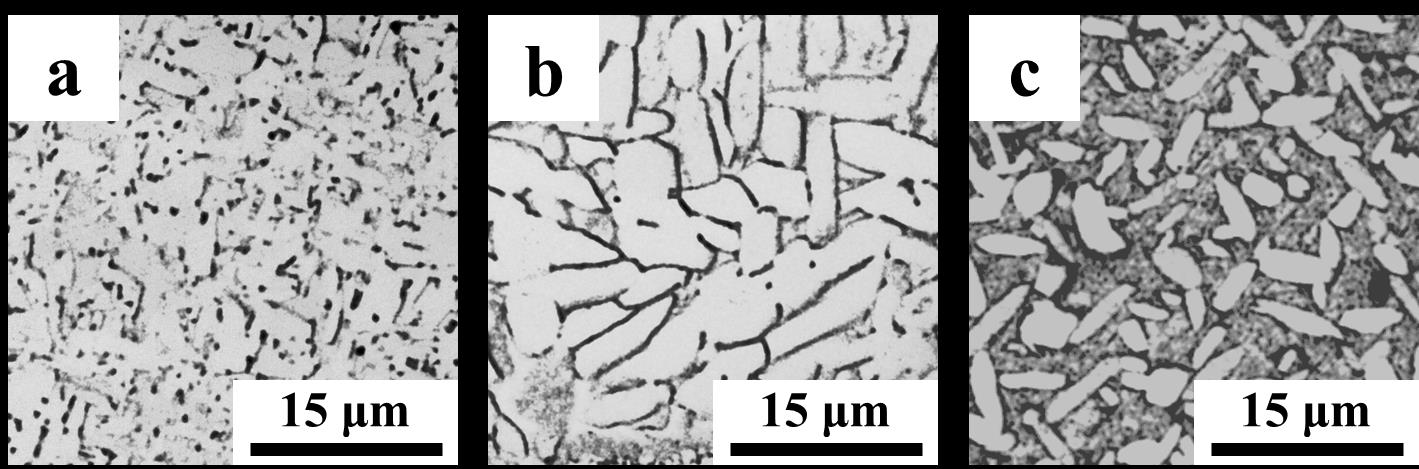
Fig 2. Optical micrographs of Ti-6Al-4V produced via HSPT: a) as-sintered UFG basket-weave structure, b) fine globularized microstructure produced by heat treatment with furnace cooling, and c) bi-modal microstructure produced by heat treatment with water quenching. The light phase is a-Ti and the dark phase is ß-Ti.
Three optical micrographs of the microstructures that result from HSPT are shown in Figure 2 [6]. Because of the very fine features in the as-sintered and bi-modal microstructures, high resolution SEM micrographs are also shown in Figure 3. In the optical micrographs, a phase shows up as light contrast and ß phase shows up as dark contrast. This relationship is reversed in SEM micrographs (a phase is dark, while ß phase is light). As shown, the as-sintered material that results from HSPT sintering and dehydrogenation (Figure 1-a) has an UFG basket-weave type microstructure (Figure 2-a and Figure 3-a). When the material is heat treated with fast cooling/water quenching and ageing (Figure 1-c), it results in a bi-modal microstructure. The bi-modal microstructure consists of about 50 vol% globularized primary a grains that form during the high temperature hold, and the remainder consists of acicular a grains and a small fraction of retained ß that form during cooling (Figure 2-b and Figure 3-c). The last structure shown is the globularized structure that forms via heat treatment with slow cooling/furnace cooling followed by ageing (Figure 1-d). All the a phase in this structure is globularized with retained ß existing only at the triple points of the a grains (Figure 2-c).
Table 1. Typical mechanical properties of Ti-6Al-4V produced via HSPT with and without heat treatment. For reference, typical mechanical properties of wrought Ti-6Al-4V after various heat treatments [7] as well as the ASTM standard for Grade 5 Ti-6Al-4V [5] are also given.
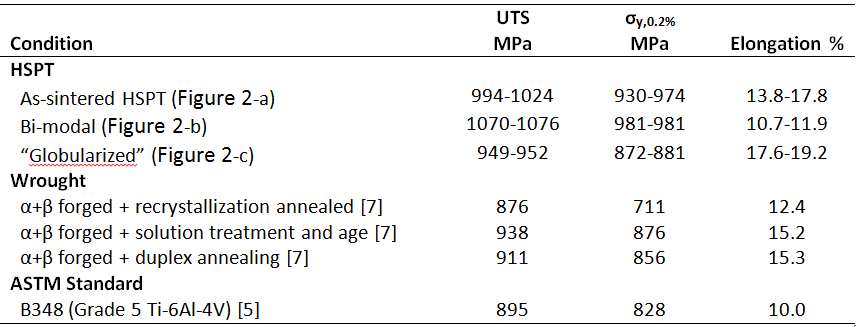
Typical ranges of mechanical properties for the three HSPT-produced Ti-6Al-4V microstructures shown in Figure 2 are given in Table 1 [6]. For comparison, typical mechanical properties for Ti-6Al-4V produced via wrought processing [7] as well as the ASTM B348 standard for grade 5 Ti-6Al-4V are also given [5]. As shown, the strength and ductility can be varied significantly by applying different heat treatments to HSPT-produced Ti-6Al-4V. For each condition, the mechanical properties are either comparable or superior to a similar microstructure produced via wrought processing. Additionally, while the mechanical properties of the HSPT-produced material varies widely with respect to processing, the strength and ductility for each condition exceed the ASTM standard. Therefore, these data demonstrate the viability of HSPT to be a replacement for wrought processing, as this process is capable of producing application-tailored mechanical properties. Ti-6Al-4V is a very popular titanium alloy for structural applications. This is because this alloy is capable of having a range of mechanical properties, meaning the material can be engineered to have mechanical properties that are tailored for the application. There, this ability is important for any prospective alternative to wrought processing for producing Ti-6Al-4V.
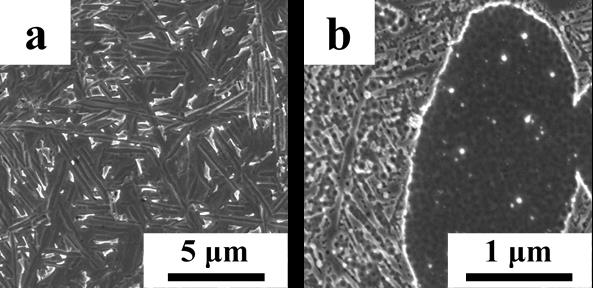
Fig 3. High resolution SEM micrographs of Ti-6Al-4V produced via HSPT: a) as-sintered UFG basket-weave structure, and b) bi-modal microstructure produced by heat treatment with water quenching. The dark phase is a-Ti and the light phase is ß-Ti.
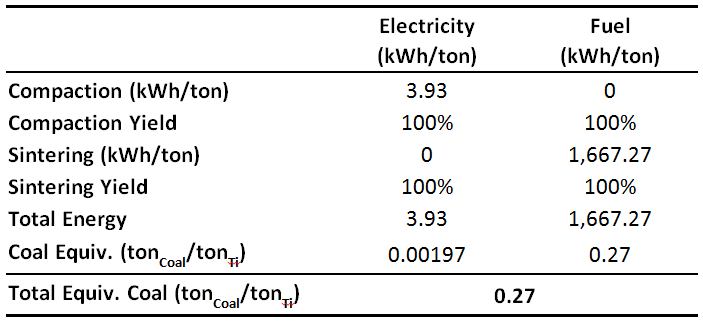
Because HSPT has been developed as a low-cost alternative to wrought processing, the energy requirements of this process in an industrial application have been quantitatively modeled and compared to wrought processing [1,8]. The energy requirements for producing Ti alloy is strongly dependent on the specific product being produced. Therefore, for this energy model, a typical mill product of 2” round bar stock was chosen. Because the energy consumption of an industrial wrought process is generally guarded proprietary information, these values were also calculated as a comparison for this model assuming a typical gyratory forging process for producing bar stock. The findings of this model are given in Table 2. It should be noted that a significant economic benefit of PM processing is its near-net-shape (NNS) capability. Therefore, modeling the energy consumption of producing a mill product such as bar stock does not account for the cost savings associated with NNS processing. However, despite this fact, this conservative energy model still predicts that HSPT will have an approximate 80% savings in energy consumption versus wrought processing.
Table 2. Calculated energy consumed per ton to produce round bar stock with a 2 inch diameter via HSPT versus wrought processing [8].
Acknowledgements
This research is supported by the US Department of Energy, Innovative Manufacturing Initiative (DEEE0005761), through the Advanced Manufacturing Office and the Office of Energy Efficiency and Renewable Energy. Raw materials are provided by Reading Alloys (AMETEK).
References
- J.D. Paramore, Z.Z. Fang, P. Sun, in:, M. Qian, F.H. (Sam) Froes (Eds.), Titan. Powder Metall., 1st ed., Butterworth-Heinemann, Oxford, 2015, pp. 163–182.
- P. Sun, Z.Z. Fang, M. Koopman, J. Paramore, K.S.R. Chandran, R. Yang, J. Lu, Acta Mater. 84 (2015) 29.
- Z.Z. Fang, P. Sun, Key Eng. Mater. 520 (2012) 15.
- G. Lütjering, J.C. Williams, Titanium, 2nd ed., Springer, Berlin Heidelberg New York, 2003.
- ASTM Standard B348-10, Standard Specification for Titanium and Titanium Alloy Bars and Billets, ASTM, West Conshohocken, PA, 2010.
- J.D. Paramore, Z.Z. Fang, P. Sun, M. Koopman, K.S.R. Chandran, M. Dunstan, Scr. Mater. ACCEPTED (2015).
- R. Boyer, G. Welsch, E.W. Collings, Materials Properties Handbook - Titanium Alloys, ASM International, 1994.
- J. Paramore, Z.Z. Fang, Unpublished (n.d.).
